
Remote Defence Access Service (RDA) – Toronto South Detention Centre - The Criminal Lawyers' Association (CLA)
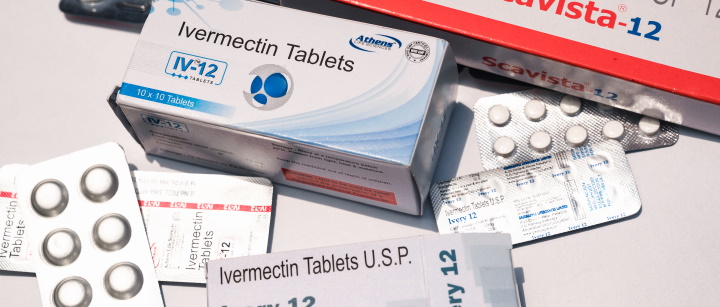
Ongoing Clinical Trials Will Decide Whether (or Not) Ivermectin Is Safe, Effective for COVID-19 - FactCheck.org
POPIS ODOBRENIH KLINIČKIH ISPITIVANJA U REPUBLICI HRVATSKOJ U 2020. GODINI 1. „Djelotvornost i sigurnost profilaktičke primj

Coronavirus vaccine update: With no vaccine ready, drugs are being tested world over for immediate treatment of Covid-19 | India News - Times of India











